Page 2
Quality Resource Guide –
Infection Control and OSHA Update Part One 3rd Edition
www.metdental.com
Guidelines and Regulations
I
t is important to realize that the Occupational
Safety and Health Administration (OSHA) and
the Center for Disease Control (CDC) are two
completely different governmental agencies with
different mandates (Table 1). The CDC develops
guidelines designed to protect both the patient
and the HCW, while OSHA regulations apply only
to the latter. Guidelines published by the CDC or
other advisory agencies do not carry the weight
of law possessed by a regulatory agency such
as OSHA. OSHA has the authority to require and
enforce compliance with recommended infection
control practices and procedures. OSHA relies
upon appropriate authorities, including the CDC,
to provide background information when they
formulate their standards. It is important that
dental providers be aware of updates or changes
to recommended infection control practices to
provide the safest environment possible for their
patients and employees, as well as to remain in
compliance with OSHA regulations.
Governmental regulations from federal agencies
such as the OSHA, and state and local health
departments, require the HCW to be trained
in appropriate infection control practices and
other safety precautions. They also require
application of these measures during patient care
to reduce potential risks of disease transmission
to the patient and the HCW. The development
of a specific set of OSHA regulations to protect
the HCW from occupational risks associated
with bloodborne disease transmission began
in the 1980’s when unions representing HCWs
petitioned OSHA to require employers to have
a workplace free from recognized harm. More
specifically, unions wanted employers to protect
employees from occupational HBV infection. After
a series of public hearings, OSHA published the
Bloodborne Pathogens Standard on December
6, 1991.
3
These regulations were based on
CDC universal precautions recommendations
and went into effect in early 1992.
4
The OSHA
standard imposed obligations on employers to
provide safe and healthful work environments
for all HCWs. Requirements included work
practice controls, engineering controls, personal
protective equipment, and administrative controls.
In the dental setting these controls can be
described as:
1. work practice controls relating to the manner in
which a task is performed and advising the use
of safer work practices designed to minimize
the risk of disease transmission;
2. engineering controls that are technology-based
(refer to items or instruments that isolate a
hazard, such as a sharp’s disposal container);
3. personal protective equipment including the
use of gloves, masks, protective eyewear, and
protective clothing to prevent contamination
of the HCW during the delivery of dental care.
4. administrative
controls
(the
policies,
procedures and practices within a dental office
that reduce risks associated with bloodborne
disease transmission).
Revisions to the Bloodborne Pathogens Standard
were mandated in 2001.
5
These revisions clarified
the need for employers to consider safer needle
devices as they become available and to involve
employees directly responsible for patient care
(e.g., dentists, hygienists, and dental assistants)
in identifying and choosing such devices.
Engineering controls are available which can be
used as the primary method to reduce exposures
to bloodborne pathogens. The controls include
sharps containers, self-sheathing needles, safety
scalpels with retractable blades or covers, as
well as safer medical devices, such as sharps
with engineered sharps injury protection and
needleless systems. Dental anesthetic syringes
and needles that incorporate safety features have
been developed for dental procedures, and their
implementation and routine use in dental facilities
is increasing.
Standard Precautions
I
nfection control recommendations for
dentistry have routinely focused on the use of
universal precautions (UP). These precautions
were designed to prevent the transmission of
HBV, HIV, HCV and other bloodborne pathogens
during treatment procedures. While the adoption
and routine use of UP proved to be very successful
in minimizing the potential for transmission
of bloodborne pathogens, these practices did
not eliminate the need to address disease-
specific isolation precautions for non-bloodborne
infections in outpatient settings.
A body substance isolation system (BSI) was
proposed in 1987
6
that focused on the reduction
of transmission of infectious materials from any
moist body substances. The BSI was designed
to address isolation procedures of all moist,
potentially infectious body substances regardless
of their presumed infectious status. The BSI
system protocol advocated additional protection
for the HCW, including immunization against
selected infectious diseases transmitted by
airborne or droplet modalities (measles, mumps,
rubella, varicella) and the use of appropriate
barriers (protective clothing).
CDC developed and published new guidelines
for isolation precautions in hospitals in 1996
7
,
in an effort to prevent any potential infectious
problems that might arise as a result of the
confusion between BSI and UP. The 1996
guidelines incorporated the major features
of UP and BSI. Since that time, the use of
Standard Precautions has replaced the use of
both of its individual components. Standard
Precautions apply to contact with blood, body
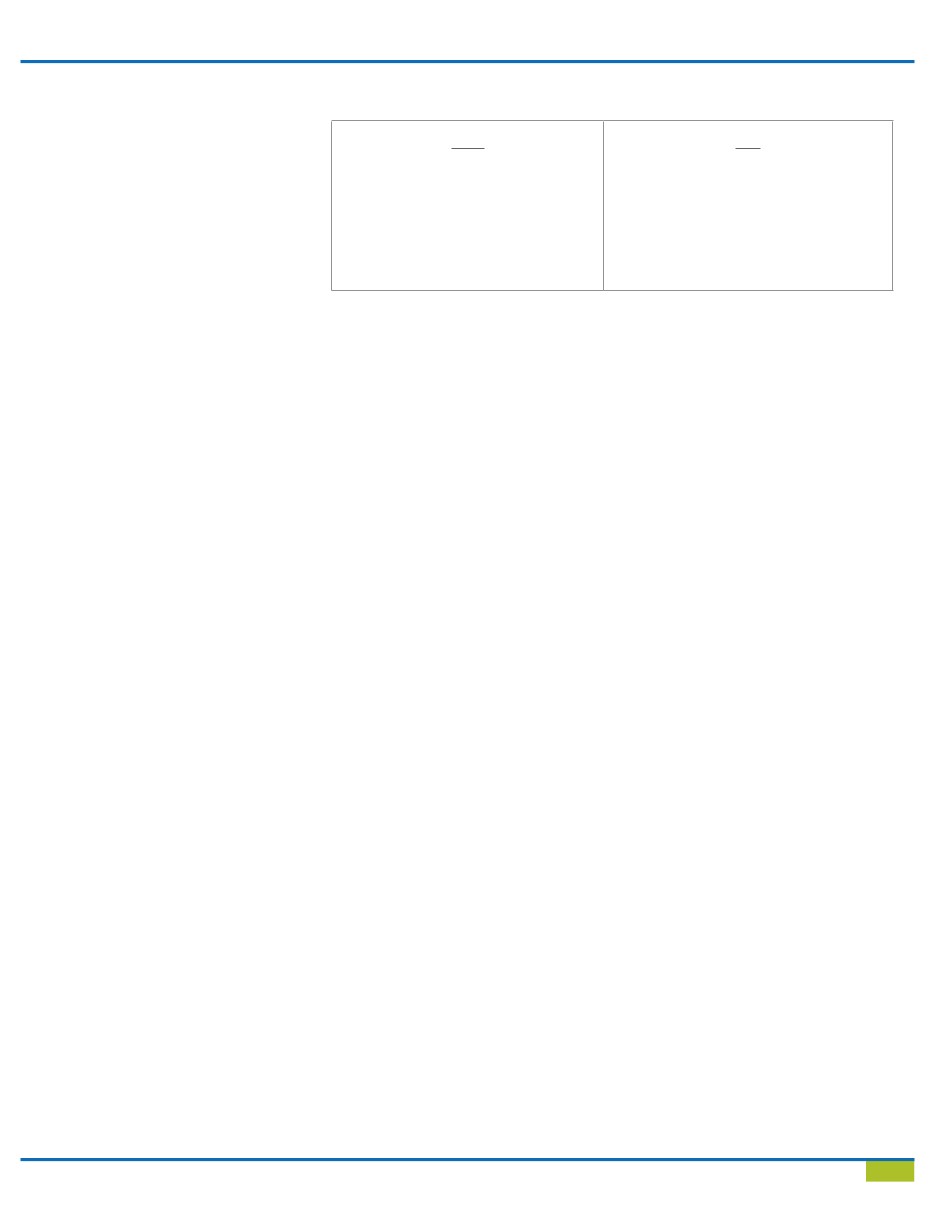
Table 1 - OSHA Regulations vs. CDC Recommendations
OSHA
•
Regulatory agency
•
Set and enforce standards
•
Investigates and inspects
•
Blood-borne Pathogen Standard
29 CFR 1910.1030 and CPL 2-2.69
•
Employee protection
CDC
•
Non-regulatory agency
•
Guidelines/Recommendations
•
Morbidity and Mortality Weekly Report
Recommendations and Reports
•
Often enforced by state